CTPS1
Step Pharma is a pioneer of CTPS1 inhibition, a new approach with the potential to yield a highly selective, safe and effective cancer treatment for both blood cancers and solid tumours.
Watch this short video to find out more about our lead CTPS1 selective inhibitor, dencatistat, and our breakthrough science.
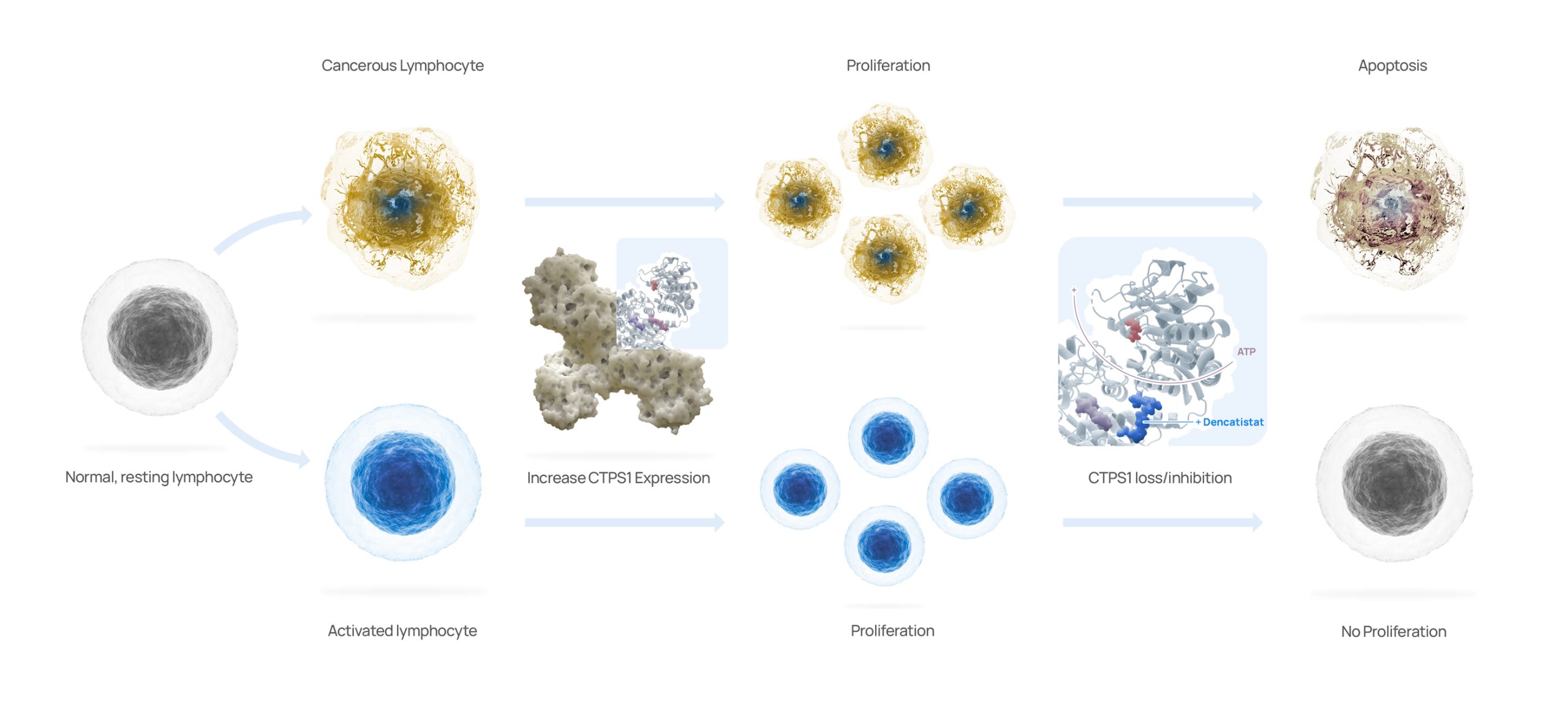
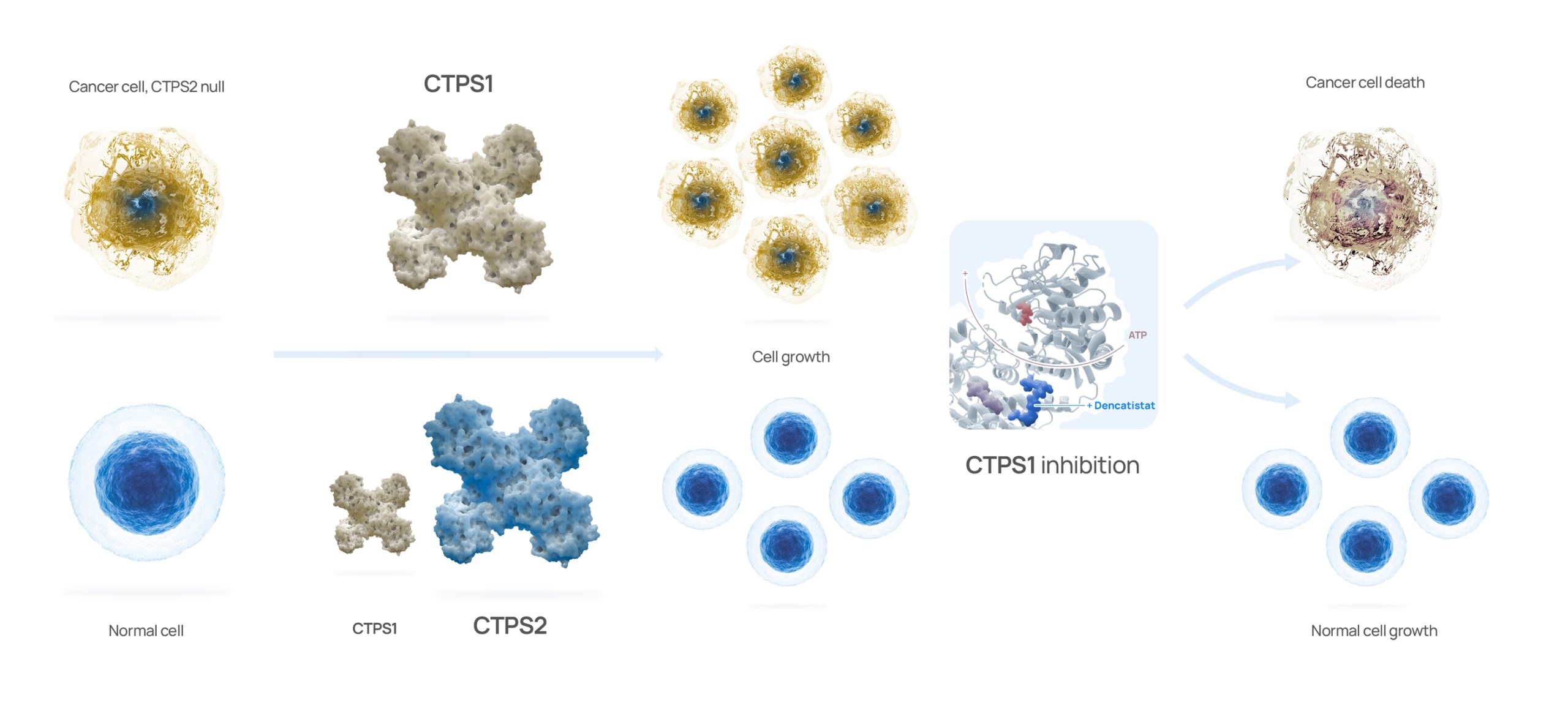
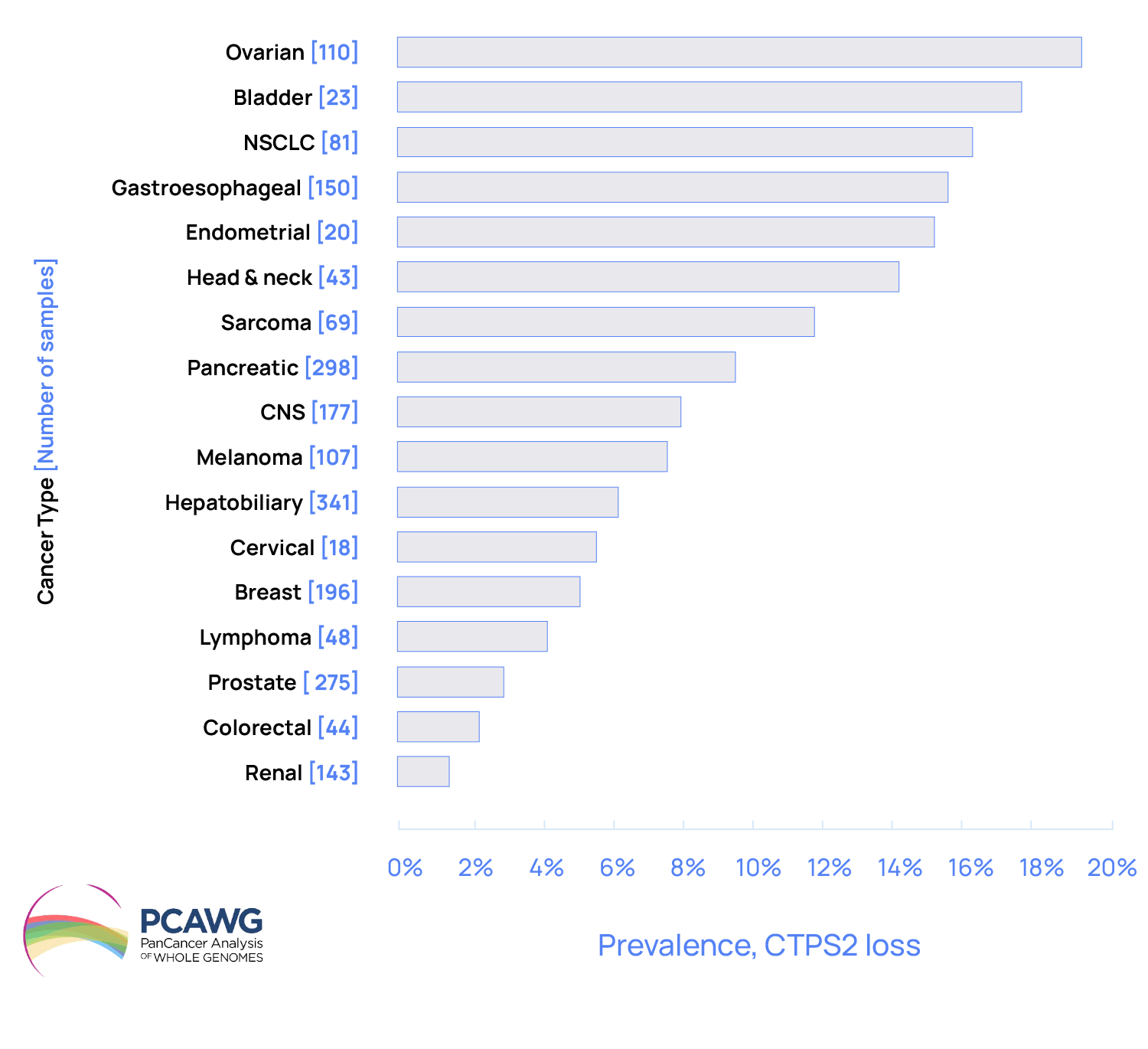
Step Pharma has unlocked the de novo pyrimidine synthesis pathway – a challenge which has eluded cancer researchers for years. We have demonstrated that all cancers exhibit a dependency on CTPS1 and that genomic loss of CTPS2 dependency, indicating that Step Pharma’s approach may be used to treat many different types of cancer.
Our novel approach could see the highly selective treatment of cancer without the significant side effects associated with current and emerging cancer therapies.
Driven by our deep understanding of CTPS1 biology and nucleotide synthesis, Step Pharma has discovered dencatistat, a first-in-class, highly selective, orally bioavailable CTPS1 inhibitor. It is a targeted therapy that kills cancer cells while sparing healthy cells
Inhibited CTPS1
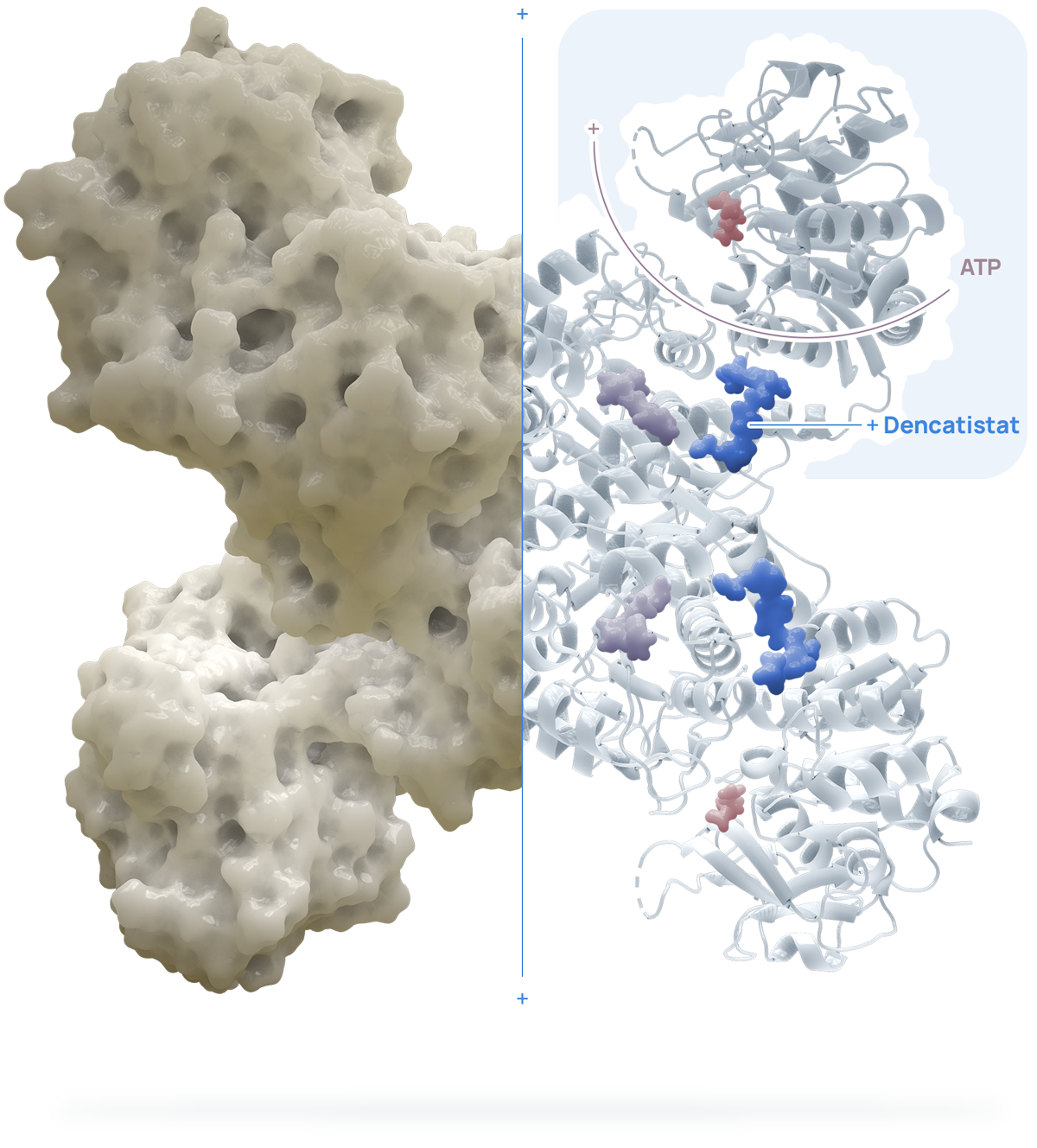
Dencatistat is a potent, highly selective, orally bioavailable CTPS1 inhibitor with greater than a 1,000 selectivity for human CTPS1 over CTPS2. Dencatistat binds to each monomer of CTPS1 mimicking CTP and thus inhibits the enzymatic activity of CTPS1.
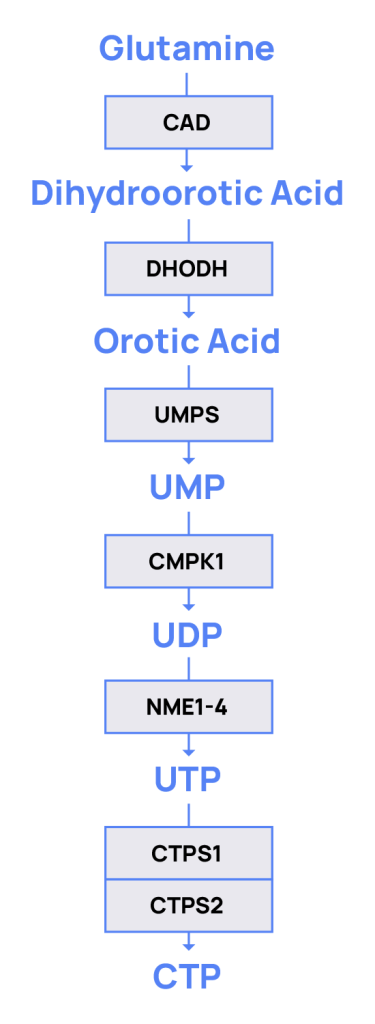
The de novo pyrimidine synthesis pathway.
CTPS1 in Solid Tumours
Prevalence of CTPS2 loss across different cancer types
Driving a step change in the treatment of cancer for patients.
DNA and RNA synthesis are essential metabolic processes to support cell growth and division.
01 I 07
Cancer cells have an increased proliferation rate and nucleotide synthesis is a known therapeutic vulnerability.
02 I 07
Historically drugs targeting inhibition of pyrimidine synthesis (PALA, DHODH, CPEC) have failed primarily due to dose limiting toxicity.
03 I 07
De novo synthesis of cytidine triphosphate (CTP) is required for DNA replication.
04 I 07
The rate limiting step is conversion of UTP to CTP which can be catalyzed by CTP Synthase 1 (CTPS1) and CTPS2.
05 I 07
All cancer cell lines appear addicted to CTPS1 for pyrimidine synthesis whereas CTPS2 is not essential.
06 I 07
This provides a point of differentiation versus previous attempts to block pyrimidine synthesis based upon the relative importance of CTPS1 vs CTPS2 in cancer biology.
07 I 07
3D crystal structure showing dencatistat bound to CTPS1.
Dencatistat
Our lead CTPS1 inhibitor dencatistat has the potential to become a key treatment for a multitude of cancers.
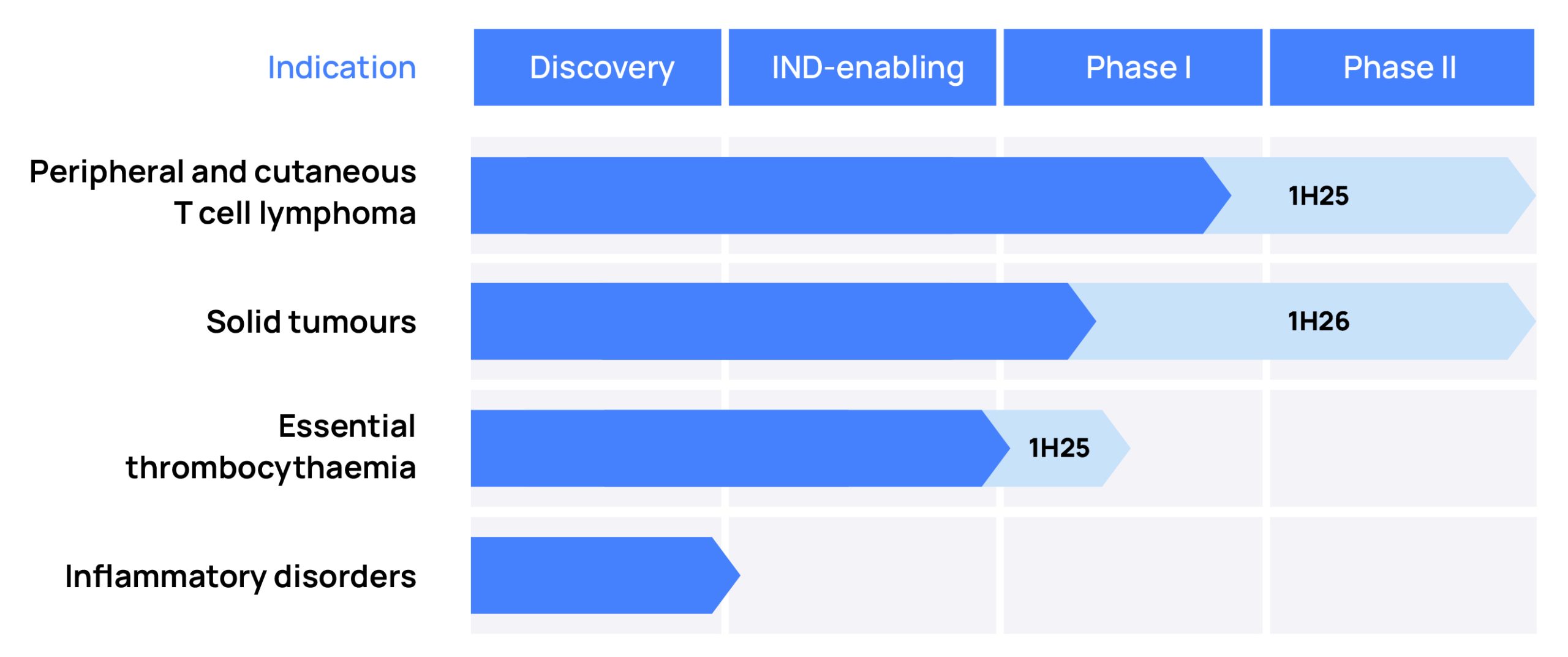
Dencatistat is in clinical development for the treatment of blood cancers, with the potential for accelerated approval from a single arm phase 2 study. Dencatistat is also in clinical development for solid tumours with the initial focus on ovarian cancer. In the future dencatistat could also form the backbone of various treatment regimens for solid tumours.
Dencatistat, a pipeline in a product

Step Pharma
15 rue Louis et Auguste Lumière
Technoparc du Pays-de-Gex
01630 Saint-Genis-Pouilly
France